Kiem Trinh1Sung, Wang-Chou2
1. Vietnam Institute on Toxicology (VIT), Email: trinhkiem0211@gmail.com
2. Taiwan National Health Research Institute, Email: sung23@nhri.edu.tw
I. INTRODUCTION
1) The ecological characteristics of Vietnam, Laos and Cambodia are very favorable for the development of venomous snakes.
Vietnam (VN) is an agricultural and tropical country with a population of 97.261.435 people (United Nations data, April, 2019), located on the S-shaped strip of land in the Indochina peninsula. Which is determined from the 102o8’ to 109o27’ Meridian East, and from the 8o27’ to 23o23’ Latitude North. VN has a land area of 331.699 km2 and the East Sea is more than 1 million km2, surrounded by 3.260 km long coastline. The length of VN from Nam Quan to Ca Mau cape is 1.650 km. The width of the narrowest place is Quang Binh, only 50 km. There is Bachma mountain 1.444 m high with Haivan pass which is part of Truong Son mountain range that cuts directly to the East Sea. This topography has created 2 different climate zones: The North has 4 distinct seasons: Spring, Summer, Autumn and Winter. The Hoang Lien Son mountain range has many bows along with the valley of the Red, Lo, Da and Thai Binh rivers creating the Red river Delta with an area of 15.000 km2, where is the largest population in VN.
The South is determined from Hai Van pass to cape Ca Mau with the Central highlands and two geographical regions (East and West) with 2 distinct seasons: Dry season (from December to April) and Rainy season (from May to November). Average temperature for the whole year is (21-28oC). Temperature fluctuates all year round (5oC). Average annual rainfall is (from 1.500 to 2.000 mm) with moisture (80-82%). This is a very favorable environment for poisonous snakes to develop.
The East is a highland area (Average: 200 m compared to the West), with rubber forests, pepper and cashew trees. It is here that the Viper species are concentrated, the most dangerous is Calloselasma rhodostoma.
In the West, there is the CuuLong river with nine branches that make up the Mekong Delta as wide as 40.000 km2. This is the granary of the world. However, it is the low-lying, boggy land along the wilderness with interlaced canals. Which is a favorable place for the development of cobra species (Fig.1).
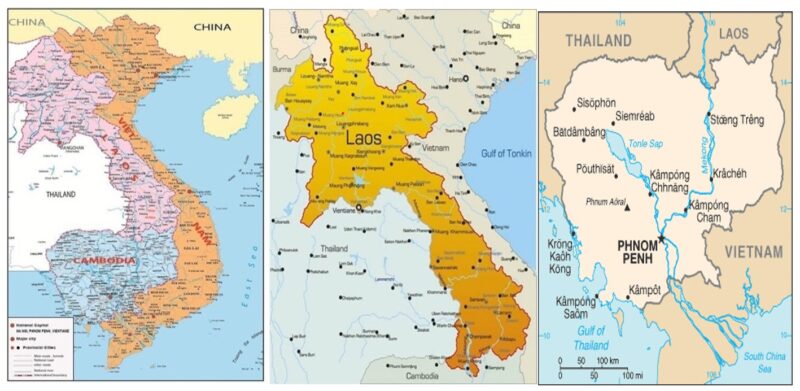
Two neighboring countries: Laos and Cambodia forming three Indochina countries with similar ecological characteristics: Laos has an area of 236.800 km2 with a population of 7.040.844 (2019), full access to the North of VN. Cambodia has an area of 181.035 km2 with a population of 16.428.267 (2019), full access to the South of VN. Therefore, venomous snakes in Laos are identical to those found in Northern VN and similarly venomous snakes in Cambodia are identical to those in Southern VN.
2) The accidents caused by snake bites and AV production in Vietnam.
WHO (2019). Dr Tedros Adhanom Ghebreyesus Director-General wrote: “Snakebites are a significant risk to health and well-being for 5.8 billion people around the world, and for those affected carry a high financial burden that often cannot be met.” [28]
WHO (2023): “A high-quality antivenom provides the best available treatment for approximately 5.4 million people who are bitten by snakes each year. Safe, effective antivenoms could prevent many of the 83.000-138.000 deaths caused by snakebites and reduce the severity of serious disabilities that impact many thousands more victims”. WHO has listed snakebite envenoming as a highest priority neglected tropical disease and launched the snakebite envenoming roadmap with the goal to halve the global burden of snakebite by 2030 [27, 29].
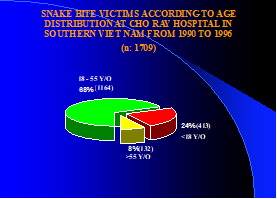
According to the results of an epidemiological investigation under the Ministry of Health of VN, there are about 30.000 (Thirty thousand) snakebite victims each year in VN. Most of these victims were the main workers of each family (Fig.2). With reference to research method of Hung DZ. (2004): Taiwan’s venomous snakebite and Blessmann J’s research, also have similar results as VN [3, 9].
The mortality rate in the severe envenoming snakebite group was very high (19.5%) because there was no AV. Although it’s more than 100 years after the invention of AV by Dr A. Calmette right at Pasture Institute in Saigon, VN [1, 2]. Meeting urgent clinical requirements, Dr. Kiem Trinh and his colleagues of the Venomous Snake Research Unit, have opened a new history of AV manufacturing and successful clinical application in VN [13, 14, 18, 22].
As the result, more than 3,000 (three thousand) victims of severe snake venom poisoning were saved by AVs made by Vietnamese people. The mortality rate of victims of severe snake venom poisoning has decreased to 1.5% [16].
However, the scale of AV manufacturing is still limited to the laboratory. The shortage of AV in VN is very serious. It is in great need of the attention of the State, the help of domestic, international organizations and individuals to develop AV production on an industrial scale, promptly meeting the requirements of saving the lives of thousands of snakebite victims in Vietnam. This is the goal of the AV production research program that the (VIT) has been focusing all our energy and wisdom on for many years.
II. METHODS
2.1. Method of identification and classification of venomous snakes in Vietnam.
According to the method for family and species identification of venomous snakes, Prof. Volfgang Wuster &. A. Malhotra, Ecology, Evolutionary biology University of Aberdeen, UK and Prof. D.Warrell [5, 30]. We had a good opportunity to collaborate on a survey of venomous snakes throughout VN. There are 12 basic criteria that need to be defined. Example of identifying Calloselasma rhodostoma according to the following standards below:
1) Kingdom: Animalia.
2) Phylum: Chordata.
3) Class: Reptilalia.
4) Order: Squamata, Scab.
5) Suborder: Serpentes.
6) Family: Viperidae.
7) Subfamily: Crotalinae.
8) Genus: Calloselasma.
9) Species: Rhodostoma.
10) Common name (English name): Malayan Pit Viper).
11) Scientific name: (Calloselasma rhodostoma).
12) Vietnamese name: Choam Quạp (The name was given by Kiem Trinh, the Snake Research Unit, Cho Ray hospital, 1990). [2].
2.2. Antivenom manufacturing method in Vietnam
According to the Guidance of WHO and experts in AV manufacturing, we carried out the fabrication at AV according to the following the technical process (Diagram.1) [5, 10, 30].
Diag.1: Summary the technical process of AV manufacturing in Vietnam.
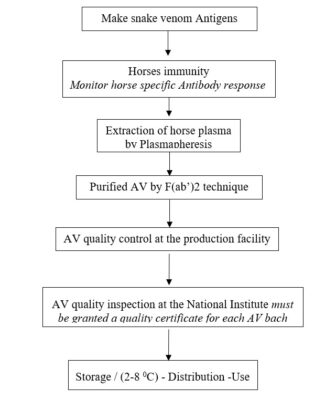
a) Make snake venom antigen
After identifying and classifying typical snakes from each geographical area, it is necessary to select healthy snakes of different ages. Milking the venom, centrifuge, freeze dried and then store in (2-8 oC). Making snake venom Antigen (Ag) according to D.Theakston’s technique by using glutaraldehyde to detoxify the venom [23]. Ag was divided into doses suitable for the purpose to stimulate the horse to produce the highest antibody (Ab), but the horse remains healthy.
b) Equine immunity produces specific antibody with the highest titer.
Select at least 03 healthy horses for each type of AV. Administered subcutaneously a dose of snake venom Ag according to a definite schedule at monthly intervals (Fig.3). Monitor horses for antibody reproductive response by using Ouchterlony technique. [29].
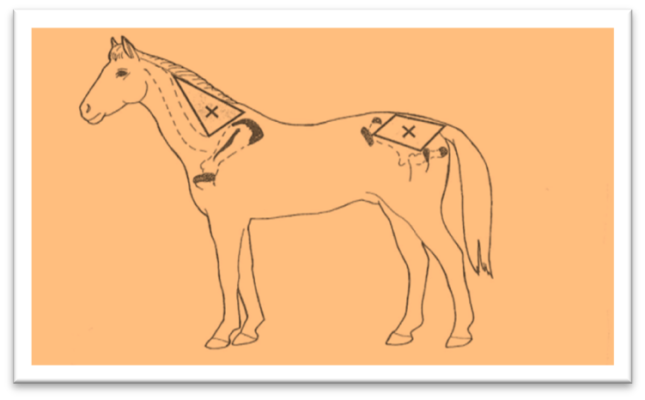
c) Extraction of horse plasma by plasmapheresis technique.
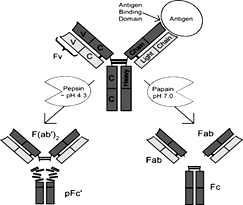
d) Purification of AV by F(ab’)2 (Fig.4) [5,10, 29].
e) AV Quality control at the manufacture factory and at the National Institute for Quality Control of Vaccine and Bioproduct, VN Ministry of Health.
According to the main standards prescribed by the Vietnam Pharmacopoeia V of Antivenom, Ministry of Health, 2018 [26]. The AV produced by Vietnam Institute on Toxicology (VIT) must be quality tested at the manufacturing facility to achieve good results. Next, the AV must be checked by the National Institute for Quality Control of Vaccine and Bioproduct, VN Ministry of Health, then issue a certificate for each batch. There are 7 standards as follows:
- Sterility: Sterility criteria were determined by the AV culture technique in 02 media: Thioglycollate and Sabouraud. After 14 days of following-up, no bacteria or mold was detected, the result is satisfactory.
- No pyrogens: This standard was defined by the following technique: Select 03 healthy rabbits, each one weighs (2-2.5 kg). Raising rabbits in a standard room for 24 hr. Measure the rabbit’s rectal temperature for 03 times, each time 01 hr apart. Required temperature difference between 03 measurements less than 1oC.
AV injection into rabbit ear vein at a dose of (0,5 ml/kg). After 03 hours of AV injection, measure the rabbit’s rectal temperature. The rabbit’s temperature requirement was increased less than 0.6 oC. Total temperature rise of all 03 rabbits must be less than 1.3 oC. The result of the pyrogen-free test was satisfactory.
- General safety test: This test was done as follows: choose 03 guinea pigs each with a weight (250-350g). Intraperitoneal AV injection in each guinea pig at a dose of 05 ml. After 07 days of follow-up, guinea pigs did not lose weight and showed no clinical sigs. General safety test result was satisfactory.
- 4. AV’s pH requirement in the range: 6,0 to 7,0.
- AV’s total protein requirement was (170 g/l). Albumin was only a trace on the electrophoresis tape.
- Preservative: Merthiolate (1/10.000), less than (0,02 %). Phenol: less than (0,025 %).
- Potency: The goal of AV manufacturing is to neutralize the snake venom in the victim’s body, so it has been strictly regulated by international units (IU). Vietnam’s pharmacopoeia stipulates:
– Determine the 50% Lethal Dose (LD50).
– Determine the Effective Dose (ED50) of each manufactured AV.
f) Cold storage AV at (2-8 oC) and distribution to medical facilities in time to treat the snakebite victims.
III. Results
3.1. The successfully identified families and species of venomous snakes in Vietnam
A. Elapidae:
- Naja naja or Naja atra (Chinese cobra): They are only available in the North of Vietnam and Laos.
- Naja kaouthia (Monocellate cobra): They are only available in the Southwest region of Vietnam and Cambodia.
- Naja siamensis (Thai spitting cobra): They are found in the Southeast, Central south of VN and Cambodia.
- Ophiophagus hannah (Yellow king cobra). They are found in the Southwest of VN and Cambodia.
- Ophiophagus hannah (Black king cobra): They are found in the Central and Northern VN, Laos and Cambodia.
- Bungarus fasciatus (Banded krait: They are available in all 3 regions of VN, Laos and Cambodia.
- Bungarus candidus (Malayan krait)): They are only available in the South of Vietnam, Laos and Cambodia.
- Bungarus multicinctus (Chinese or many banded krait): They are only available in the North of VN and Laos.
- Bungarus flaviceps (Red River Krait), (Bungarus slowinskii): They are discovered in the North of VN. However, the consequence of this snake is not clear.
B. Viperidae:
- Trimeresurus albolabris (White-lipped green pit viper): They are everywhere in VN, Laos and Cambodia.
- Agkistrodon rhodostoma or Calloselasma rhodostoma (Malayan pit viper): They live mainly in the Southeast region of VN and Cambodia.
- T. mucrosquamatus (Chinese habu, Taiwanese pit viper): Rarely see them in the border provinces of Vietnam and China.
C. Hydrophiidae (Sea snakes):
There are 17 species of sea snakes. They concentrated on the central coast of VN. The most popular is Enhydrina schistosa.
Images of venomous snakes mainly in Vietnam.

Fig.5: The main families and species of venomous snakes are circulating in VN
Note:
- Naja atra (A)
- Naja kaouthia (K)
- Naja (N.S)
- fasciatus (B.F)
- Yellow Ophiophagus hannah
- Black Ophiophagus hannah
- candidus (B.C)
- Bungarus multicinctus(B.M)
- Trimeresurus albolabris (T.A)
- Viperidae with a pit as a thermosensitive organ.
- Calloselasma rhodostoma (CR)
- Trimeresurus mucrosquamatus (T.m)
- Enhydrina schistose (E.S)
- Hydrophis cyanocinctus (H.C)
3.2. Research results on Antivenom manufacturing and clinical application in Vietnam.
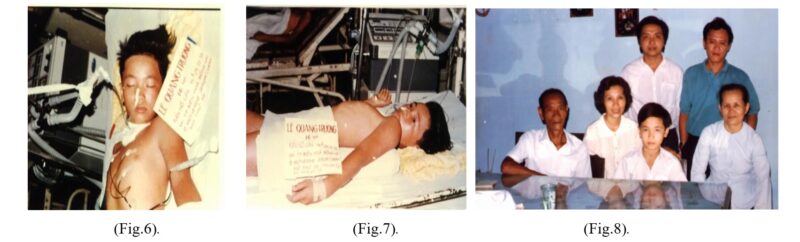
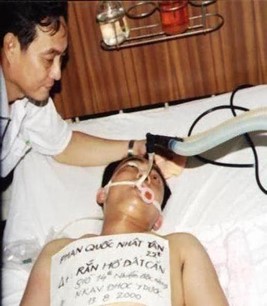
In 1991, Naja kaouthia AV was successfully manufactured by Kiem Trinh and et al, directly saving the life of Le Q T victim, who had been seriously poisoned by NK bite, to be on a ventilator for up to 58th hour at Cho Ray hospital. This was the first venomous snake victim saved by an AV made by Vietnamese people (Fig.6,7,8) [1,18].
Following that, the technical process of 05 specific AV types were studied and fully established in the laboratory. Which are:
-Technical process for making Calloselasma rhodostoma. AV [2, 21].
– Technical process for making Bungarus candidus. AV [13,14,25].
-Technical process for making Bungarus multicinctus. AV [8, 25].
– Technical process for making Yellow and Black Ophiophagus hannah. AV [17, 24].
– Technical process for making Naja siamensis.AV [20].
35 AV lots of the 6 specific AV types mentioned above have been approved by the National Institute for Control of Vaccine and Biologicals, MOH,Vietnam.
The most important result was that the AV batches manufactured by Dr. Kiem Trinh and et al of (VIT) have been clinically applied for clinical treatment according to Divid Warrell’s guidelines, directly saving the lives of over three thousand victims of severe envenoming. As a result, it has contributed to reducing the mortality rate of severe envenoming group from 19.5% to 1.5% [6], (Fig.10)
Since then, the Vietnam Society on Toxicology (VST) and Vietnam Institute on Toxicology (VIT) have been established in 2015.This is where thousands of experts and philanthropists gather to contribute to the snakebite disease has been forgotten in VN.
 |
|
|
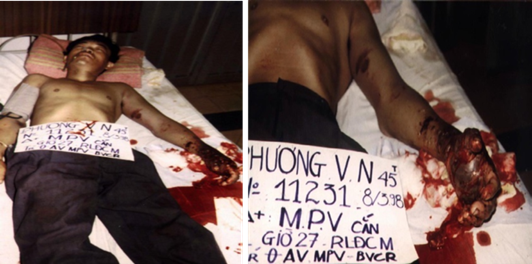
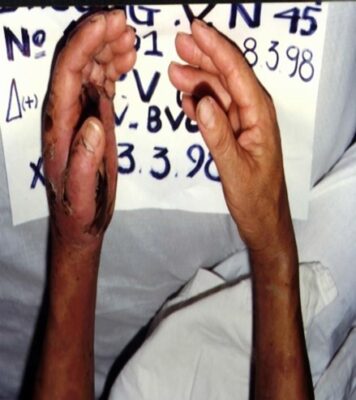
Fig.9 & Fig.10. Patient NV.Ph envenomed by Caloselasma rhodostoma (C.R) venom admitted to Cho Ray Hospital, 1998. |
 |
 |
|
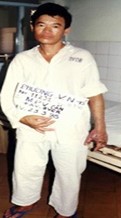
Fig.11 & Fig.12: Patient NV.Ph completely recovered from C.R bite, after treatment with specific AV at Cho Ray Hospital, 1998. |
|
 |
 |
| Fig.13 & Fig.14. PQN patient infected with Earth Tiger snake venom recovered with Earth Tiger Treatment System at Cho Ray Hospital, 1998. | |

2 patents of Calloselama rhodostoma (CR) and Naja kaouthia (NK)AV manufacturing have been granted by the State of Vietnam for technical process.
During the course of AV research and manufacturing for clinical applications, 04 PhDs and 6 Masters of medicine, specializing in venomous snakes were successfully trained. [7, 8, 20, 24, 25].
They are the experts who have been making effective contributions to the snakebite management in VN.
In December 2008, the important event was that the IST-Asia-Pacific Conference has been successfully organized by Dr. Kiem Trinh and et al in VN. It brought together more than 250 scientists around the World (Fig.11). This is the proud honor of Vietnamese toxicologists, key people continue to develop international and domestic cooperation for the success of snakebite management in VN.

IV. CONCLUSION
Snake bite is a serious tropical disease that has caused up to thirty thousand victims in Vietnam every year. The mortality rate of severe envenomed victims is up to (19.5%).
Since 1990, Dr. Kiem Trinh and the colleagues of Vietnam Institute on Toxicology (VIT) have researched and identified two venomous snake families (Elapidae and Viperidae) with 14 main venomous snake species circulating in VN.
06 main types of monospecific AVs have been successfully manufactured by VIT. They have applied to clinical applications, contributed to save the lives of over three thousand severe envenomed victims. The mortality rate has dropped to (1.5%). Once again, we have proven the strategic direction of WHO: “AV is the most effective and specific drug that has been used worldwide for many years”.
However, our researched program is still limited to the Lab. AV shortage is a serious problem in VN.
We urgently call on the State, charitable organizations, individuals from country to the World: Please take care and support the AV manufacturing program on an industrial scale for promptly and ready to rescue the snakebite victims in Vietnam, even Laos and Cambodia.
Reference
- Anh T.K, Kiem T.X et al (1997): “Research and production of Naja Kaouthia antivenom, successful clinical application in Vietnam. A project of Ministry of Health, Decision No: 1223 KHDT, 1997.
- Anh T.K, Kiem T.X et al (1997): “Research and production of Calloselasma rhodostoma antivenom, successful clinical application in Vietnam. A project of Ministry of Health, Decision No: 1224 KHDT, 1997.
- Blessmann J, Nguyen TPN, Bui TPA, et al (2018): Incidence of snakebites in 3 different geographic regions in Thua Thien Hue Province, Central Vietnam: green pit vipers and cobras cause the majority of bites. Toxicon.2018;156:61–65.doi:10.1016/j.toxicon.2018.11.009 [PubMed] [CrossRef] [Google Scholar].
- 4. Chanthawat Patikorn et al, 2021: Situation of snakebite, antivenom market and access to antivenoms in ASEAN countries. BMJ Global Health. http://dx.doi.org/10.1136/bmjgh-2021-007639.
- Chippaux JP (2010): Guidelines for the production, control and regulation of snake antivenom immunoglobulins. Biol Aujourdhui. 2010 10.1051/jbio/2009043 [PubMed] [CrossRef] [Google Scholar]
- David Warrell -WHO, August, 2016: Guidelines for the management of snakebites.
- Dong L.V (2004): Biochemical and immunological studies on snake venoms of South Vietnam. Ph.D. Thesis, National University of Singapore.
- Ha T H, Höjer J, Trinh X K, Nguyen T D (2010): “A controlled clinical trial of a novel
antivenom in patients envenomed by Bungarus multicinctus”, J Med Toxicol, Dec, 6, pp. 393-
- Hung DZ. (2004): Taiwan’s venomous snakebite: epidemiological, evolution and geographic differences. Trans R Soc Trop Med Hyg. 2004;98(2):96–101. doi: 10.1016/S0035-9203(03)00013-0 [PubMed] [CrossRef] [Google Scholar]
- Guillermo León, Álvaro Segura, Aarón Gómez, et al, 2014: Industrial Production and Quality Control of Snake Antivenoms. Living reference work entry. First Online: 01 January 2014.
11. Kiem T.X, Long X.T(2022): “Venomous snakes, from clinical practice to manufacturing anti-venom and anti-cancer bioproducts”. Vietnam Medical Publishing House, 2022.
- Kiem T X, Francis Markland Jr et al (2016): “A Novel Anti-tumor Protein from Calloselasma rhodostoma Venom in Vietnam”. Anatomy & Physiology: Curent Research.
- Kiem T.X, Quyen L.K, Long T.X, David A. Warrell (2010): “Hyponatraemia, Rhabdomyoly- sis, alterations in blood pressure and persistent mydriasis in patients envenomed by Malayan kraits (Bungarus candidus) in Southern Vietnam”, Toxicon.
- Kiem T.X, et al (2009): “Research and manufacturing Bungarus candidus antivenom, clinical application successfully in Vietnam”. Vietnam Medical Journal, No:1, 2009.
- Kiem XT; Tuyen DT; Long XT (2009): “The production of Bungarus candidus & Bungarus multicinctus antivenom from horses immunized with venom & its application for the treatment of snake bite patients in Vietnam”, The XVIth World Congress of International Society on Toxinology, Brazil.
- Kiem Trinh et al (2008): Venomous snakes and snakebite management in Vietnam. The 8th IST-Asia Pacific Meeting on Animal, Plant & Microbial Toxins organized in Vietnam, December, 2008
- Kiem T.X et al (2007): “Research to develop production Ophiophagus hanna antivenom, successful multi-center clinical application in Vietnam”. Department of Science and Technology, Ho Chi Minh City, 2008.
- Kiem XT, Quyen LK, Phuoc NB (1997), “Snake bites in Vietnam, the initial results of research and production of Naja kaouthia antivenom and its application in the treatment of victims at Cho Ray hospital, Ho Chi Minh city, since 1990”, 12th the World congress of IST on animal, plant and microbial toxins, Cuernavaca Morelos, Mexico.
- PatikornC, et al:Global systematic review of cost of illness and economic evaluation studies associated with snakebite. J Glob Health 2020; .doi:10.7189/jogh.10.020415pmid:http://www.ncbi.nlm.nih.gov/pubmed/33312499
- Quyen L K, (PhD student), Kiem Trinh (Instructor), 2022: “Research to perfect the technical process of manufacturing Naja siamensis Antivenom in Vietnam”. Ph.D. Thesis. Vietnam Military Medical Academy, 2022.
- Quyen L.K, Kiem T.X (2008): A case with brain hemorrhage caused by Calloselasma rhodostoma successful treated with C.R antivenom. The 8th IST-Asia Pacific Meeting on Animal, Plant & Microbial Toxins.
- Quyen L.K (2003): Clinical Evaluation of Snakebites in Vietnam: A Study from Cho Ray hospital. A thesis for the Degree of Master of Science. National University of Singapore.
- R.D.G.Theakston*, D.A.Warrell, E.Griffiths (2003) “Erratumto Report of WHO workshop on the standardization and control on antivenoms” [Toxicon41(5)(2003)541–557]
- Thuy T.B (Master student), Kiem T.X (2008): “Research to perfect the technical process of manufacturing Ophiophagus hannah Antivenom in Vietnam”. A thesis for the Degree of Master of Science. Vietnam Military Medical Academy, 2008.
25.Tuyen T.D (PhD student), Kiem T.X (Instructor) (2014): “Research to perfect the technical process of manufacturing Bungarus candidus Antivenom in Vietnam”. Ph.D. Thesis. Hanoi Medical University, VN, 2014.
- Vietnam Pharmacopoeia 5. Ministry of Health. Medical Publisher, 2018.
- WHO (2023): Guidelines for the management of snake-bites World Health Organization.
28.WHO (2019): Snakebite envenoming: a strategy for prevention and control. Available: https://apps.who.int/iris/bitstream/handle/10665/324838/9789241515641-eng.pdf
- 29. WHO (2010): Guidelines for the production, control and regulation of snake antivenom immunoglobulins. 134. Geneva: WHO, 2010.
- Wolfgang Wuster (1994): Ecology and evolutionary biology of venomous snakes. University of Aberdeen, UK.